The concentration of metals (ppm) in double distilled water at 60±2ºC... | Download Scientific Diagram
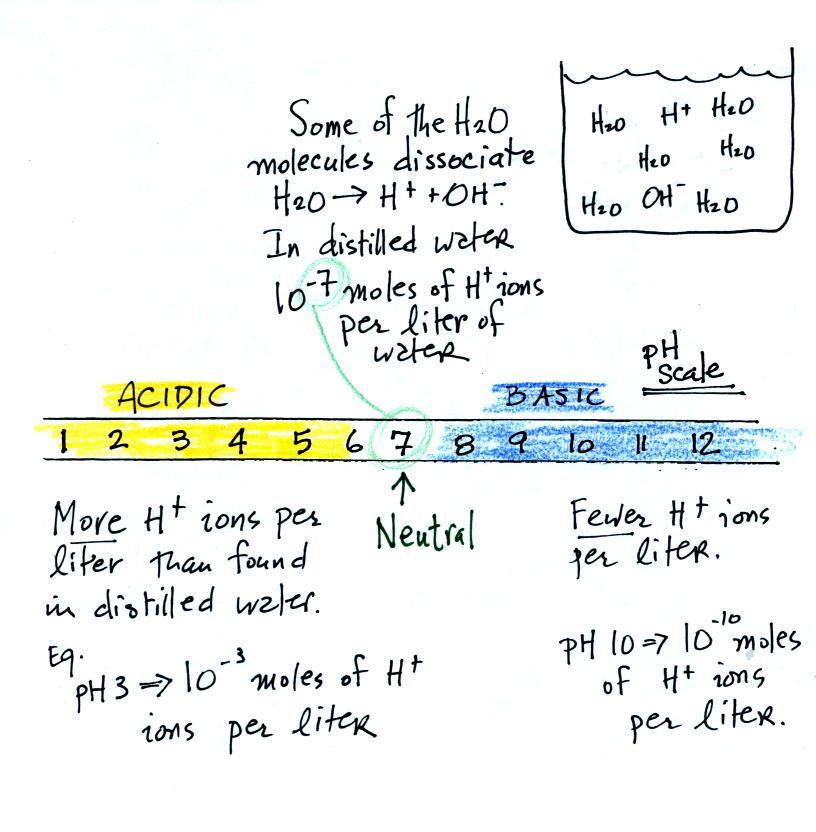
![At `80^(@)C` distilled water has `[H_(3)O^(+)]` concentration equal `\'^(+)O 1xx10^(-6) \"mole\"// - YouTube At `80^(@)C` distilled water has `[H_(3)O^(+)]` concentration equal `\'^(+)O 1xx10^(-6) \"mole\"// - YouTube](https://i.ytimg.com/vi/sG9XjpQwtSA/maxresdefault.jpg)
At `80^(@)C` distilled water has `[H_(3)O^(+)]` concentration equal `\'^(+)O 1xx10^(-6) \"mole\"// - YouTube
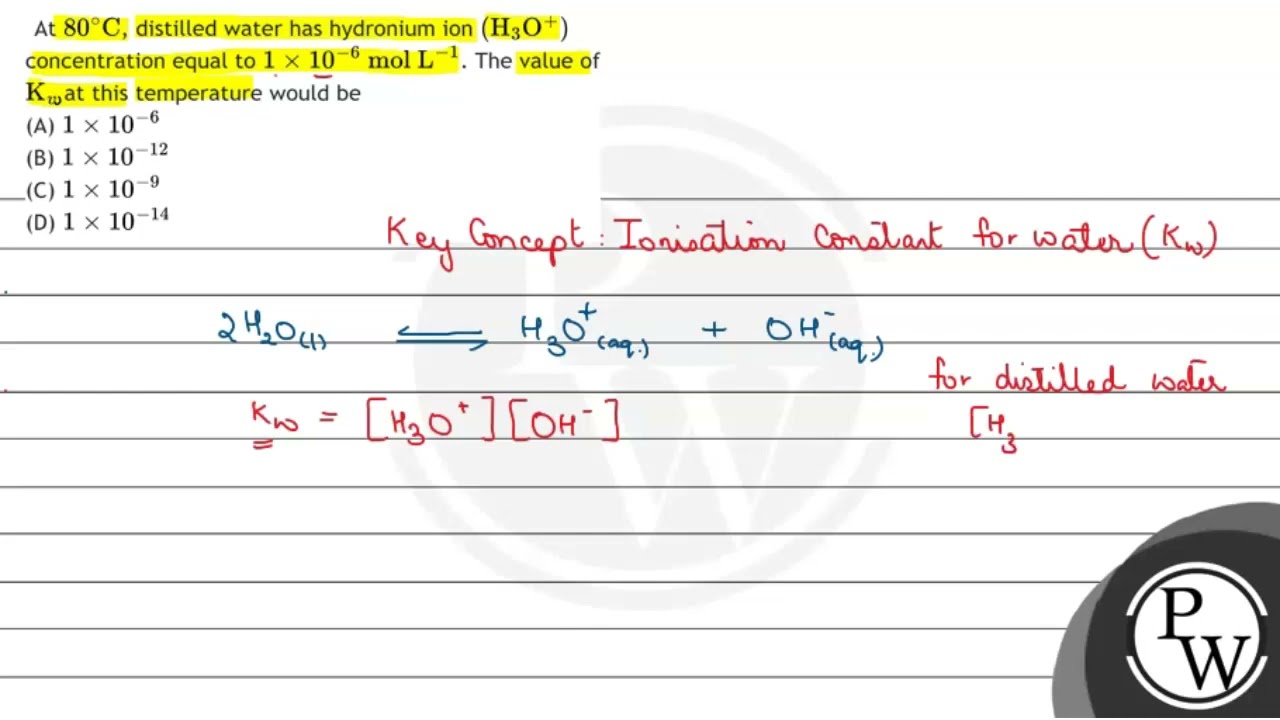
At \( 80^{\circ} \mathrm{C} \), distilled water has hydronium ion \( \left(\mathrm{H}_{3} \mathr... - YouTube

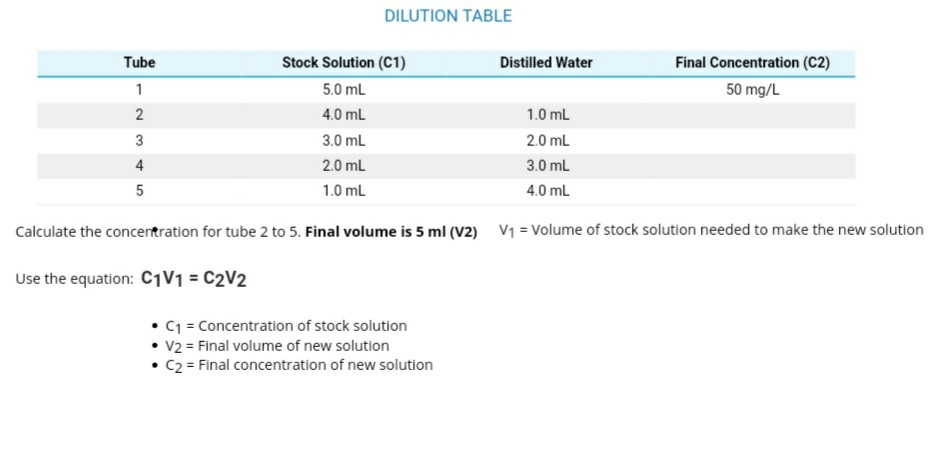
You will be performing many dilutions in this experiment. If John adds 17.51 mL of 6M HCl to 6.36 mL of - Brainly.com
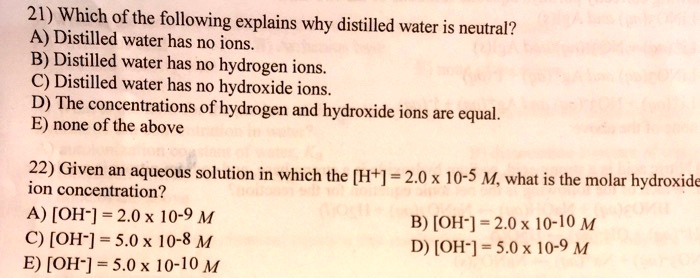
SOLVED: 21) Which of the following explains why distilled water is neutral? A) Distilled water has no ions B) Distilled water has no hydrogen ions C) Distilled water has no hydroxide ions

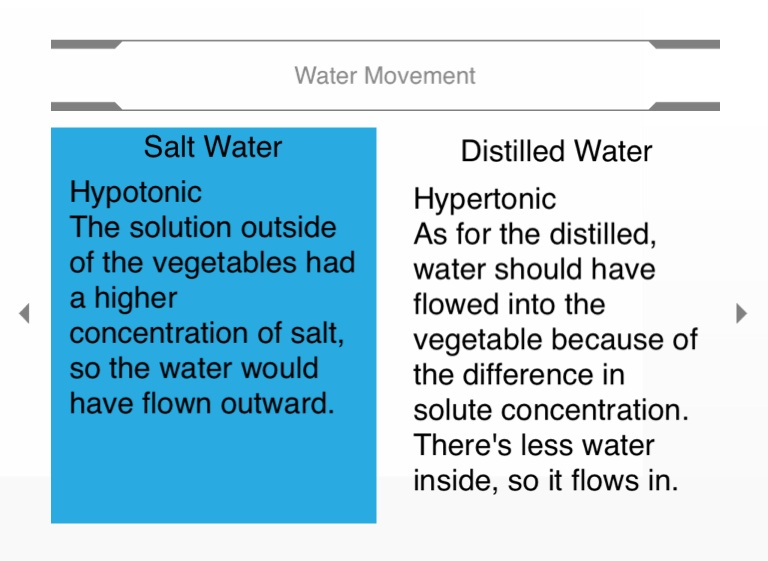
Water moved into the egg in distilled water because the higher concentration of water was outside the cell so it diffused inside t… | Glassware, Osmosis, Corn syrup
![At 80^(@)C distilled water has [H(3)O^(+)] concentration equal [OH^(-)] 1xx10^(-6) "mole"//litre. The value of K(w) at this temperature will be At 80^(@)C distilled water has [H(3)O^(+)] concentration equal [OH^(-)] 1xx10^(-6) "mole"//litre. The value of K(w) at this temperature will be](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/52405125_web.png)
At 80^(@)C distilled water has [H(3)O^(+)] concentration equal [OH^(-)] 1xx10^(-6) "mole"//litre. The value of K(w) at this temperature will be