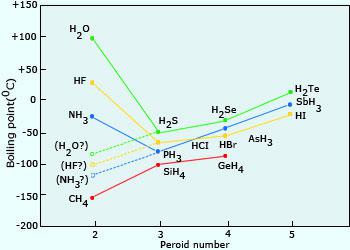
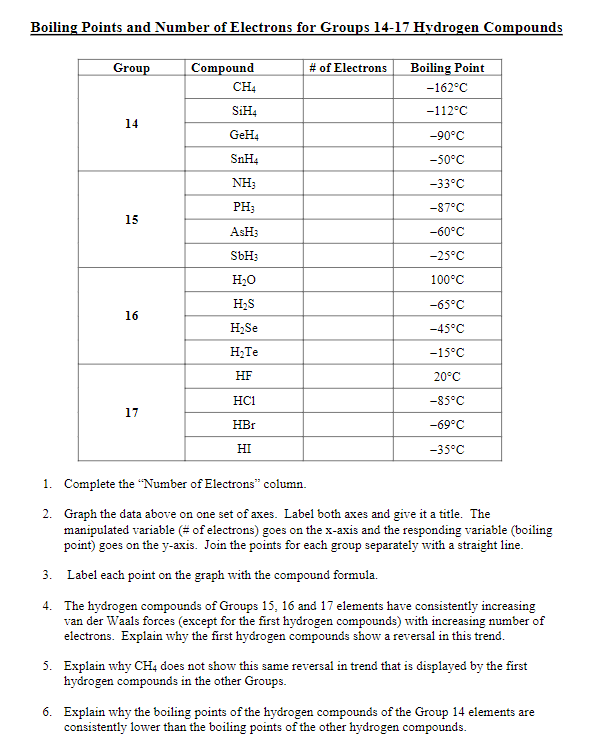
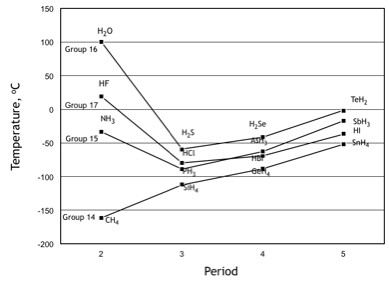
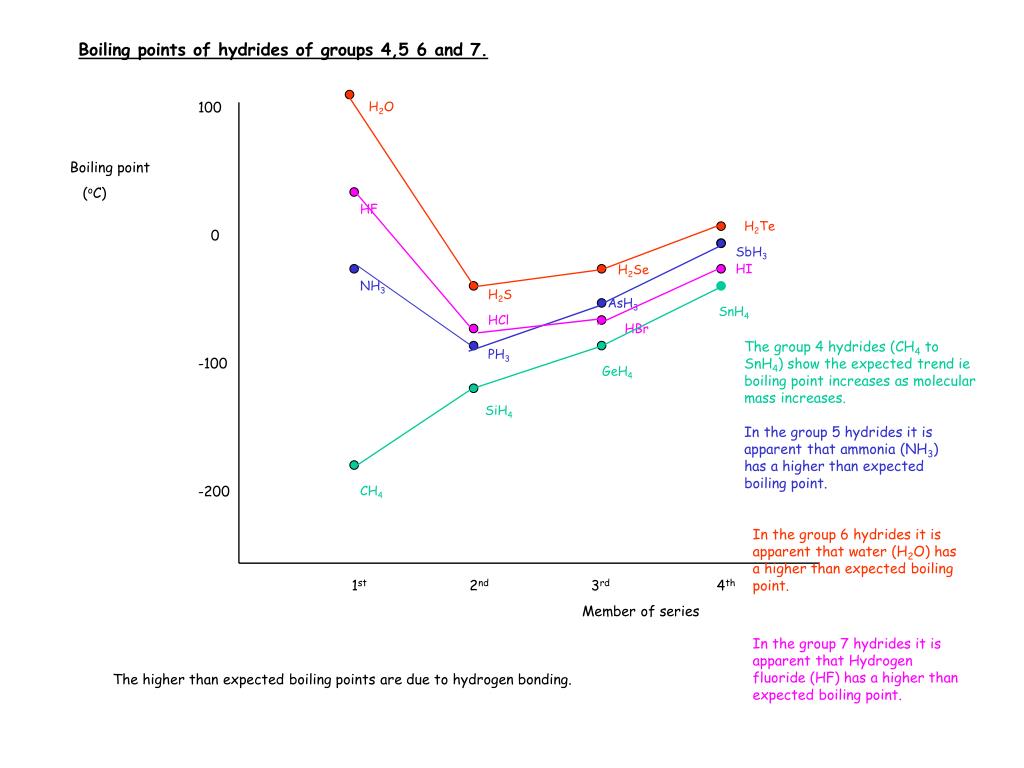
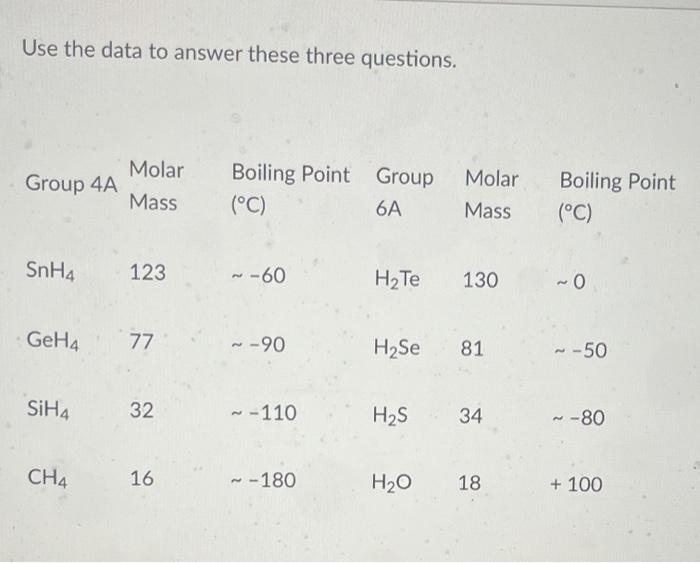
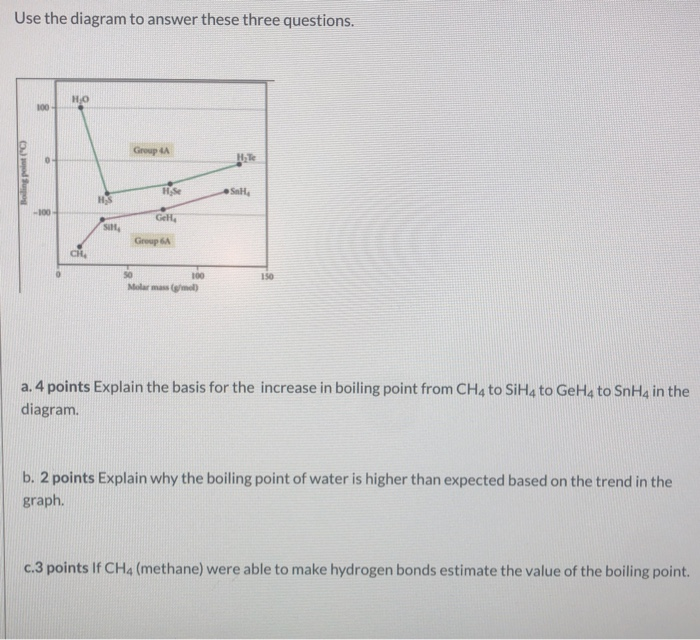
The normal boiling point of water is unusually high, compared to the boiling points of H_2S, H_2Se, and H_2Te. Explain this observation in terms of the hydrogen bonding that exists in water,
Consider the following: CH4, SiH4, GeH4, SnH4 The boiling points for these compounds increase roughly at the same rate except for CH4. Why does CH4 have a significantly lower boiling point than