Recent trends on density functional theory–assisted calculations of structures and properties of metal–organic frameworks and metal–organic frameworks-derived nanocarbons | Journal of Materials Research | Cambridge Core
Strong Relationships in Acid-Base Chemistry – Modeling Protons Based on Predictable Concentrations of Strong Ions, Total Weak Acid Concentrations, and pCO2 | PLOS ONE

First-Principle Predictions of Absolute pKa's of Organic Acids in Dimethyl Sulfoxide Solution | Journal of the American Chemical Society

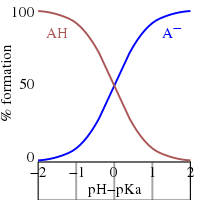
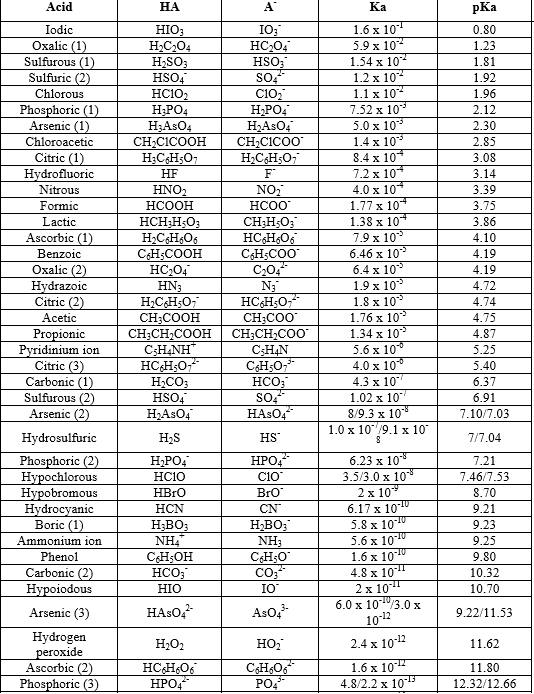
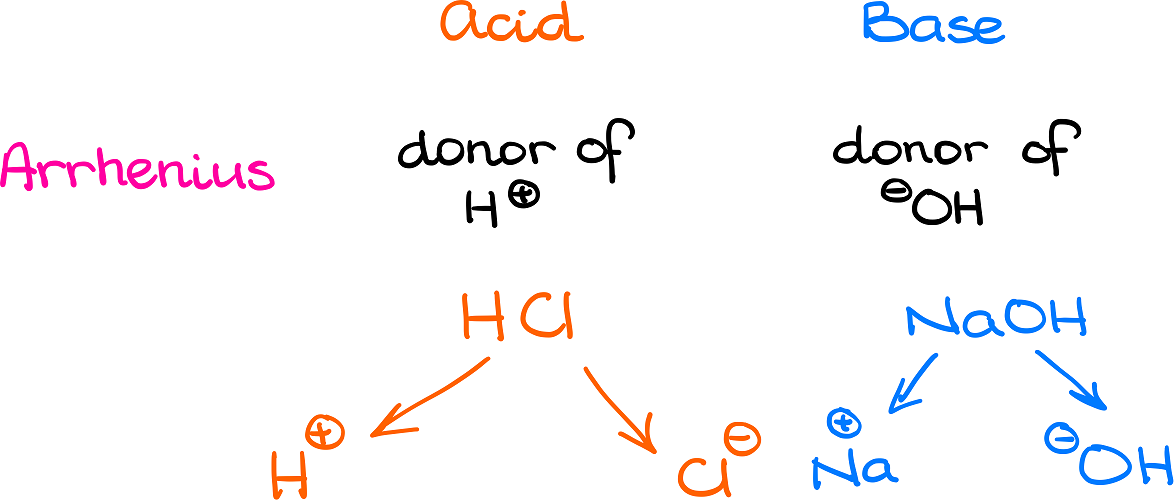
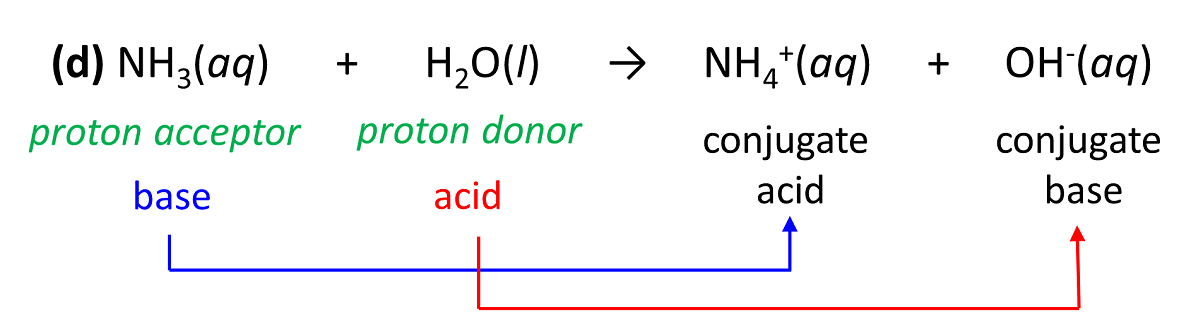
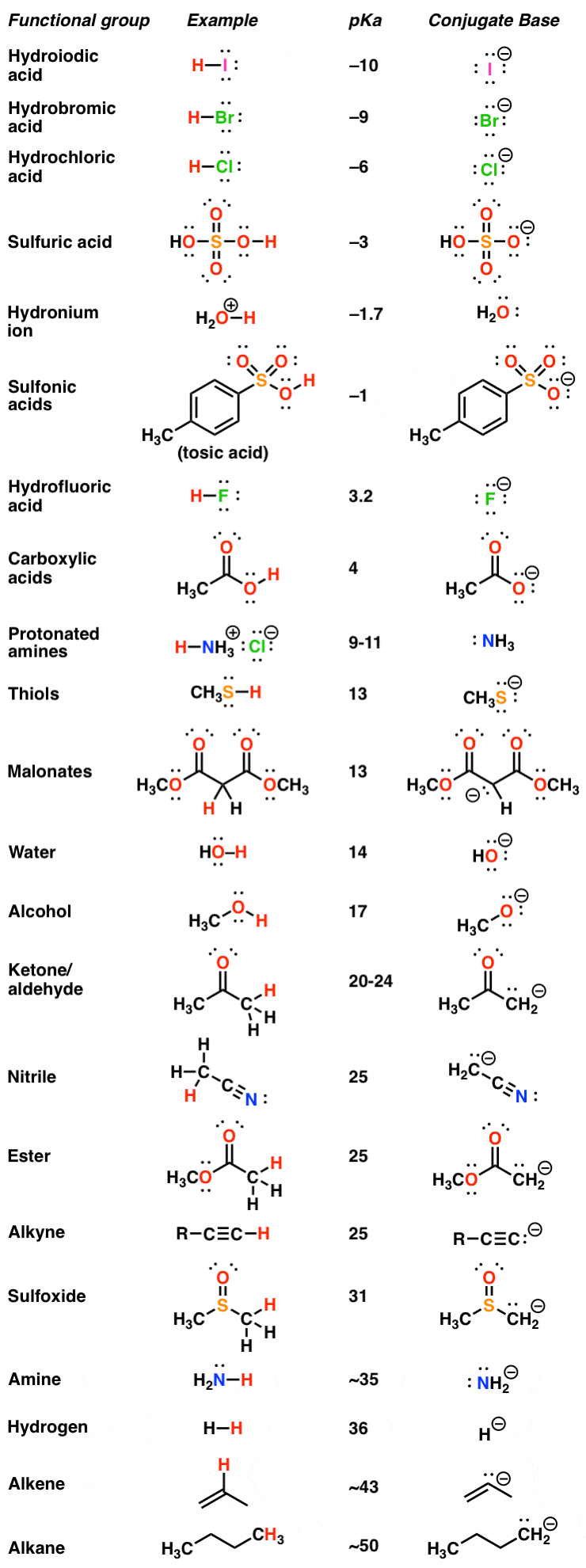
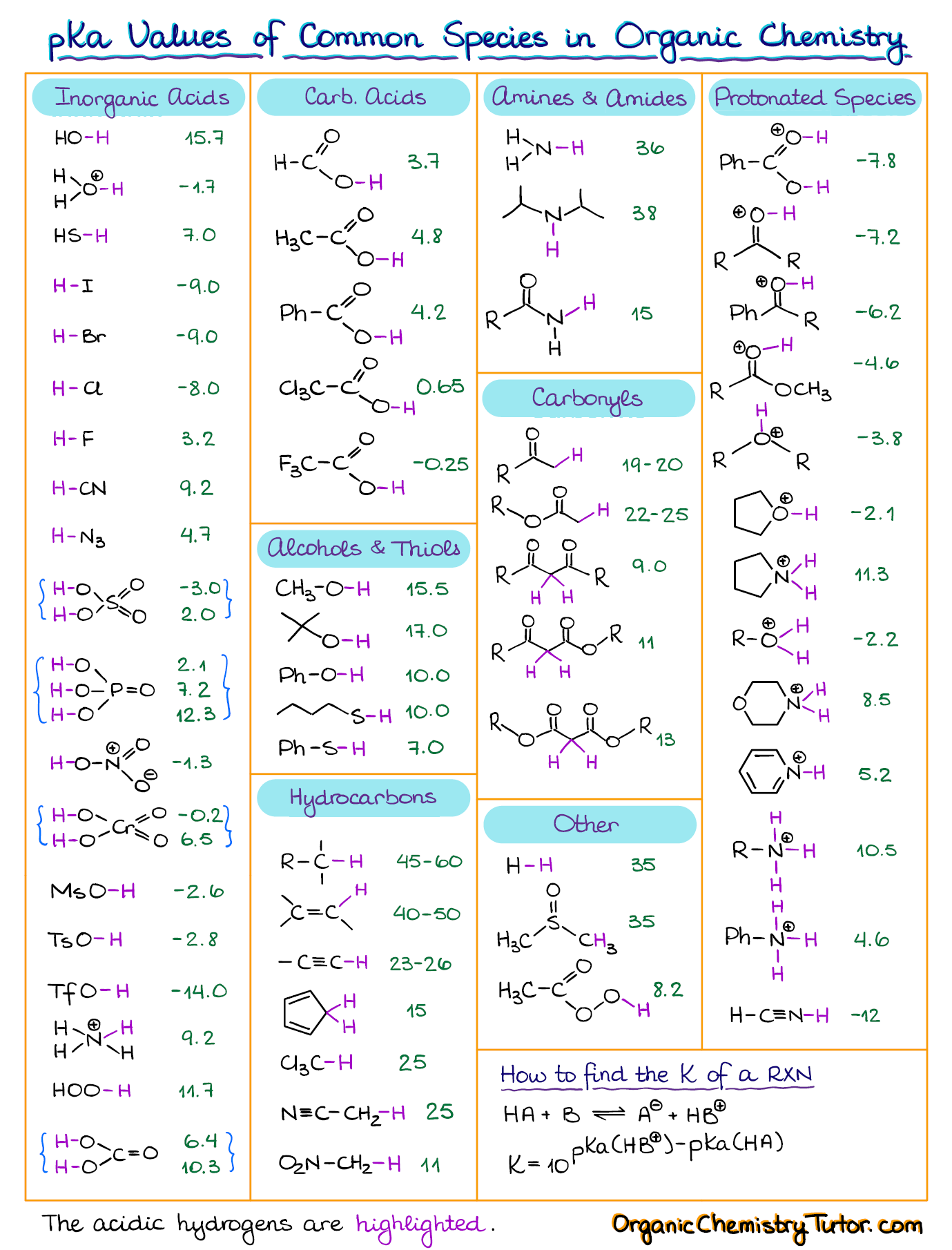
3.3: pKa of Organic Acids and Application of pKa to Predict Acid-Base Reaction Outcome - Chemistry LibreTexts

It has been found that the pH of a 0.01 M solution of an organic acid is 4.15 . Calculate the concentration of the anion, the ionization constant of the acid and its pKa .
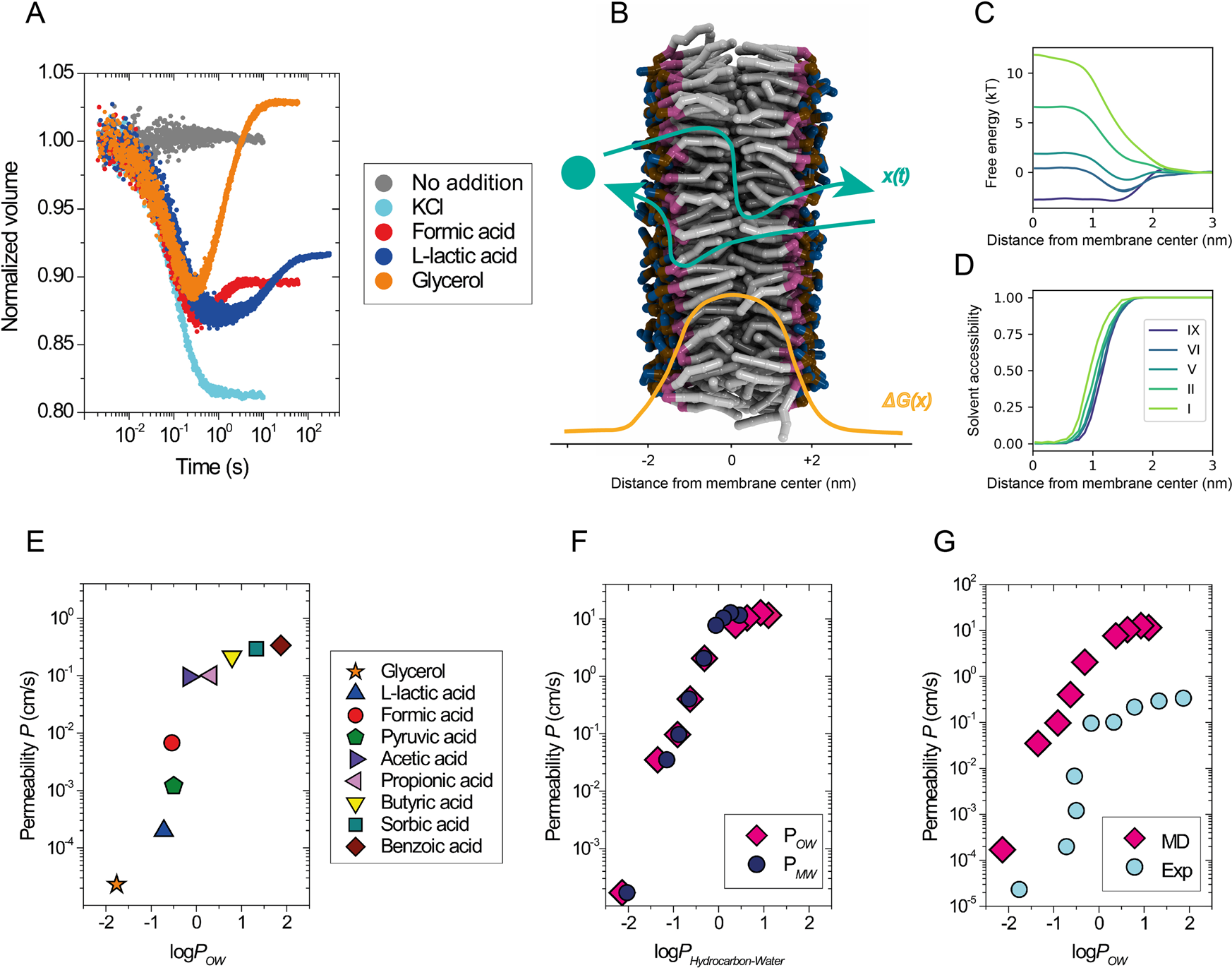
Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes | Nature Communications